An acid-base reaction is simply the transfer of a proton from an acid to a base, forming the conjugate base of the acid and the conjugate acid of the base.
For Example:
| Acid | Base | Conjugate Base | Conjugate Acid | |||
|---|---|---|---|---|---|---|
CH3COOH |
+ |
HCO3- |
|
CH3COO- |
+ |
H2CO3 |
The strength of an acid can be given quantitatively from its acid dissociation constant, Ka. For the reaction:
HA ![]() H+ + A-
H+ + A-
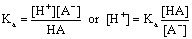
In aqueous solution, the Ka is the [H+] at which you have equal amounts of the acid and its conjugate base in equilibrium.
Because Ka can vary over many orders of magnitude, it is often expressed in logarithmic form:
pKa = -logKa
Just as [H+] is expressed in logarithmic form:
pH = -log[H+]
The larger the pKa, the weaker the acid (and the stronger its conjugate base).
Therefore, the reaction will proceed in the direction to form the acid with the larger pKa.
Click here for a table of approximate pKa values. (Values below 1 and above 16 are extrapolated from reactions measured in non-aqueous solvents.)
Examples |
||||||
|---|---|---|---|---|---|---|
CH3COOH |
+ |
NH3 |
|
CH3COO- |
+ |
NH4+ |
acid |
base |
base |
acid |
|||
pKa ~5 |
pKa~9 |
|||||
Equilibrium lies to the right: → |
||||||
C2H5NH2 |
+ |
H2O |
|
C2H5NH3+ |
OH- |
|
base |
acid |
acid |
base |
|||
pKa ~16 |
pKa ~11 |
|||||
← Equilibrium lies to the left * |
||||||
NH3 |
+ |
HCN |
NH4+ |
+ |
CN- |
|
base |
acid |
acid |
base |
|||
pKa ~9 |
pKa ~9 |
|||||
pK's the same, equilibrium has approximately equal amounts of product and reactant. |
||||||